Abstract
Background: The median age at diagnosis of patients with Chronic Lymphocytic Leukemia (CLL) is 70 years, which presents unique challenges for disease management, as these patients have more comorbidities and often require multiple concomitant medications. While some baseline characteristics (e.g., history of cardiovascular or bleeding issues) may create potential risk for significant medical events, others (e.g., hypertension, joint pain) threaten the ability of patients to stay on long-term continuous BTKi, compromising optimal therapeutic benefit. The tolerability of Bruton's tyrosine kinase inhibitors (BTKi) can be suboptimal in these patients, emphasizing the need for novel non-chemotherapy regimens with a differentiated risk profile. The combination of umbralisib and ublituximab (U2) demonstrated superior progression-free survival (PFS) and overall response rates (ORR) compared to chemoimmunotherapy in the primary analysis of the randomized, multicenter, Phase 3 UNITY-CLL trial (NCT02612311) (Gribben et al. 2020). Herein, an analysis was conducted of patients treated with U2 on UNITY-CLL who had a pre-existing comorbidity or concomitant medication that could potentially preclude the use of BTKi.
Methods: Patients ≥18 years of age with CLL who were treatment-naïve (TN) or previously treated (PT) requiring treatment per iwCLL criteria with adequate organ function and ECOG PS ≤2 were eligible. Patients were initially randomized 1:1:1:1 to receive U2, obinutuzumab+chlorambucil (O+Chl), umbralisib monotherapy, or ublituximab monotherapy. Stratification factors included treatment status (TN vs. PT) and deletion 17p (del17p) status.
Umbralisib was given orally at 800 mg once daily until progression or removal from treatment for other reasons. Ublituximab was administered intravenously at 900 mg on Days 1/2 [split 150/750 mg], 8, and 15 of Cycle 1, Day 1 of Cycles 2-6, and on Day 1 every 3 cycles after Cycle 6. The primary endpoint was independent review committee (IRC)-assessed PFS of U2 compared to O+Chl. Key secondary endpoints included IRC-assessed ORR, complete response, undetectable minimal residual disease (uMRD), duration of response, and safety, assessed from the first dose until 30 days after the last dose of study medication.
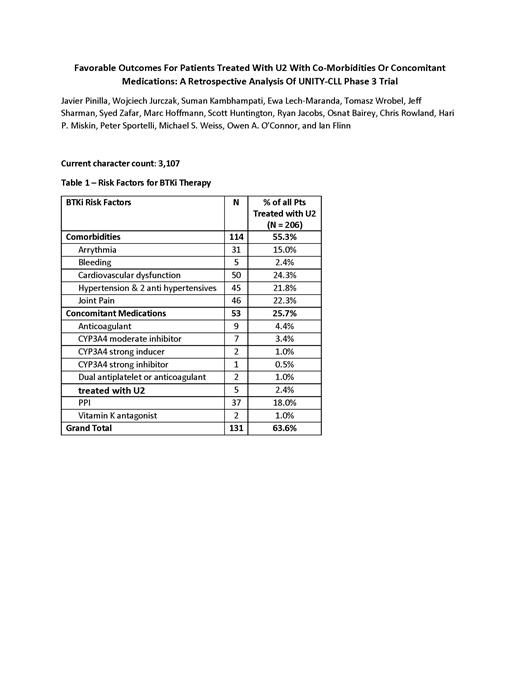
BTKi risk factors were defined by the comorbidities and concomitant medication categories listed in Table 1 and include pre-existing arrythmias, cardiovascular dysfunction, history of major bleeding, hypertension, and joint pain.
Results: In the UNITY-CLL study, 206 subjects with CLL received treatment with U2. Among these patients, the median age was 67 years, 9% had del17p, 54% had unmutated IGHV, and 57% were TN. Among these patients, 131 (63.6%) met at least 1 BTKi risk factor criteria (Table 1). Among patients with BTKi risk factors, the median age was slightly higher at 69 years, 11% had del17p, 56% were unmutated IGHV, and 57% were TN. At a median follow-up of 36.7 months, 40% remain on U2 as compared to 37% for the entire U2 population. The median PFS for patients with a BTKi risk factor was equivalent to the entire U2 population at 31.9 months, and a similar ORR (88% vs 83%, difference not statistically significant) was observed in this population as compared to the overall U2 population.
In terms of safety, there were no differences in the incidences of SAEs, grade ≥3 AEs, fatal AEs, or discontinuations due to AEs.
Conclusions: Patients with BTKi risk factors attained durable clinical benefit from U2. In this population with BTKi risk factors, the baseline disease characteristics aligned with the overall population treated with U2, and efficacy and safety outcomes did not appear to be negatively impacted by the presence of comorbidities or the use of concomitant medications. These data suggest that U2 can be a valuable treatment option for patients with these conditions.
Pinilla Ibarz: AbbVie, Janssen, AstraZeneca, Novartis, TG Therapeutics, Takeda: Consultancy, Other: Advisory; Sellas: Other: ), patents/royalties/other intellectual property; AbbVie, Janssen, AstraZeneca, Takeda: Speakers Bureau; MEI, Sunesis: Research Funding. Jurczak: Abbvie: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celtrion: Research Funding; Celgene: Research Funding; Debbiopharm: Research Funding; Epizyme: Research Funding; Incyte: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Mei Pharma: Research Funding; Morphosys: Research Funding; Novo Nordisk: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sandoz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding. Lech Maranda: Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board, lectures; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board, lectures; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board, lectures; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board, lectures; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board, lectures; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board, lectures. Wróbel: BeiGene: Honoraria; Takeda: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; BMS: Honoraria; Roche: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Speakers Bureau. Sharman: AstraZeneca: Consultancy; TG Therapeutics: Consultancy; Centessa: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an AbbVie Company: Consultancy; Lilly: Consultancy; BMS: Consultancy; BeiGene: Consultancy; AbbVie: Consultancy. Hoffmann: TG Therapeutics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pharmcyclics: Consultancy, Honoraria; celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Huntington: Flatiron Health Inc.: Consultancy; DTRM Biopharm: Research Funding; TG Therapeutics: Research Funding; AstraZeneca: Consultancy, Honoraria; Novartis: Consultancy; AbbVie: Consultancy; SeaGen: Consultancy; Genentech: Consultancy; Servier: Consultancy; Bayer: Honoraria; Thyme Inc: Consultancy; Pharmacyclics: Consultancy, Honoraria; Celgene: Consultancy, Research Funding. Jacobs: Adaptive Biotechnologies: Consultancy; ADC Therapeutics: Consultancy; SecuraBio: Consultancy, Speakers Bureau; Genentech: Consultancy; Jannsen: Speakers Bureau; TG Therapeutics: Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding, Speakers Bureau; Verastem: Consultancy; AbbVie: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; TeneoBio: Research Funding; MEI Pharma: Research Funding. Rowland: TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Miskin: TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Sportelli: TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Weiss: TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. O'Connor: Myeloid Therapeutics: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Dren: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Kymera: Consultancy, Current equity holder in publicly-traded company; TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company; Nomocan: Consultancy; Mundipharma: Consultancy. Flinn: Agios: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Trillium Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Juno Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Iksuda Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Novartis: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Forma Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Incyte: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Loxo: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Karyopharm Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Great Point Partners: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; MorphoSys: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Kite, a Gilead Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Curis: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; AbbVie: Consultancy, Other: All Consultancy and Research Funding payments made to Sarah Cannon Research Institute, Research Funding; ArQule: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Unum Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Calithera Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Pfizer: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Constellation Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Teva: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Gilead Sciences: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; BeiGene: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Genentech: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Acerta Pharma: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Celgene: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; IGM Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Seagen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Roche: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Verastem: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; AstraZeneca: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Merck: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Nurix Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Infinity Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Janssen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Takeda: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; TG Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Rhizen Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Portola Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Forty Seven: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Triphase Research & Development Corp.: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Century Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Hutchison MediPharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Vincerx Pharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Sarah Cannon Research Institute: Current Employment; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Seagen: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Unum Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute, Research Funding; Johnson & Johnson: Current holder of individual stocks in a privately-held company; Seattle Genetics: Research Funding.